cGMP Current Good Manufacturing Practices for Pharmaceuticals
Author(s) :Potdar
|

|
| ISBN |
: |
9788188449781 |
| Name |
: |
cGMP Current Good Manufacturing Practices for Pharmaceuticals |
| Price |
: |
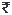 750.00 750.00 |
| Author/s |
: |
Potdar |
| Type |
: |
Text Book |
| Pages |
: |
798 |
| Year of Publication |
: |
Rpt. 2015 |
| Publisher |
: |
PharmaMed Press/BSP Books |
| Binding |
: |
Paperback |
|
|
BUY NOW |
|
Like us on our Pages






|
Book Review Form
|
|
About the Book
Indian pharmaceutical industry is becoming global at high pace. This resulted in high pressure on the industry for quality products and practices. Thus cGMP has acclaimed Key position.
Practicing cGMP requires clear understanding at conceptual and implementation level and that too at shop floor and middle management level. This book is written in simple and easy to implement manner.
Salient features
· All current issues of cGMP demanded by regulatory authorities like W.H.O. M.H.R.A., T.G.A., U.S.F.D.A, and also Indian F.D.A. are fully discussed, profusely referenced and thoroughly illustrated in simple and easy to understand language. · Covers other important topics like, plant, site security, environmental issues, distribution of products, and preparing for the regulatory audits etc. |
Contents 1. Personnel, 2. Surroundings, Buildings and Facilities, 3. Equipment, 4. Materials Management, 5. Quality Management, 6. Manufacturing Operations and Control, 7. Documentation and Records, 8. Pharmaceutical Validation, 9. Outsourcing, 10. Post-Operational Activities, 11. Sterile Pharmaceutical Products, 12. Site and Plant Security, 13. Safety and Environmental Protection, 14. Good Pharmaceutical Wholesaling Practices, 15. Pharmaceutical Audit |
About the Author
Manohar A. Potdar, M. Pharm, PhD (Production Management) USA is Professor Emeritus of Pharmaceutical Sciences at Poona College of Pharmacy, Bharati Vidyapeeth University, Pune. He teaches undergraduates and postgraduate courses on Pharmaceutical Technology, Plant design & Operation and Quality Assurance Techniques. Guides research students in the field of Pharmaceutical Validation, Quality Assurance, Plant Design etc.
He has more than 35 years of Industrial experience gained from Indian and Multinational firms like, Hoechst, Boehringer-Knoll, Burroughs Wellcome, Ranbaxy, Lupin, Wockhardt, Plethico, Cadila and Alkem, He has handled various responsibilities in the area of Q.C. / Q. A., Production, Project Management besides executing International Regulatory Audits of WHO, TGA, MHRA, MCC and USFDA. With his Postgraduate Diploma in Training and Development from I.S.T.D. (India) background, he has actively participated in many industrial training programmes. Potdar is a Member and Chairperson for many International Seminars on R.F.I.D applications in Pharmaceutical Industry. Please browse his website: www.cgmp.co.in for complete particulars. |
|
|
|

